NEWS
-
Revealing the testing principle of urine iodine detector
2024-09-30 -
What are the causes of thyroid disease
2024-08-30 -
The significance of blood iodine detection in patients with gastric cancer
2024-06-28 -
Study on the relationship between serum iodine and iodine nutritional status and thyroid function in children and pregnant women
2024-05-30 -
hazards of iodine deficiency
2024-04-18 -
Iodine detector tell you wether iodine is deficient
2024-02-27 -
Iodine not only for intelligence
2024-01-31
WHO ARE WE?
We specialize in research development and manufacture of automatic iodine detectors, and also focusing on development production of urine iodine, blood iodine, salt iodine, water iodine and other iodine detection and analysis reagents. So far, we totally own 12 patents and profession articles.
PRODUCT CENTER
-

Automatic Iodine Detector DAT50SG (CDC)(Disease Control)
Automatic Iodine Detector DAT50SG (CDC)(Disease Control)
For medical and health, disease prevention institutions, commodity inspection and quality inspection, scientific research institutions, etc.;Exclusive use of 8-point calibration to…
more>> -

Automatic Iodine Detector DAT50SG (hosp)(health checkup centers & hospital departments)
Automatic Iodine Detector DAT50SG (hosp)(health checkup centers & hospital departments)
For large provincial hospitals, maternal and child specialists, and health checkup centers,use of 6-point calibration to test 50 sample sites
more>> -

Automatic Iodine Detector DAT30SG (hosp)(health checkup centers & hospital departments)
Automatic Iodine Detector DAT30SG (hosp)(health checkup centers & hospital departments)
For clinical laboratory of large and medium-sized municipal hospitals,use of 5-point calibration to test 30 sample sites
more>> -

Automatic Iodine Detector DAT20SG (CHC)(Community Clinic)
Automatic Iodine Detector DAT20SG (CHC)(Community Clinic)
For specific population at community clinics in county-level towns,use of 4-point calibration to test 20 sample sites
more>>
TESTING REAGENT CENTER
HONORARY CERTIFICATE
-

CE CONFORMITY CERTIFICATE
...
-

High Technology Enterprise Recognition Certificate
...
-
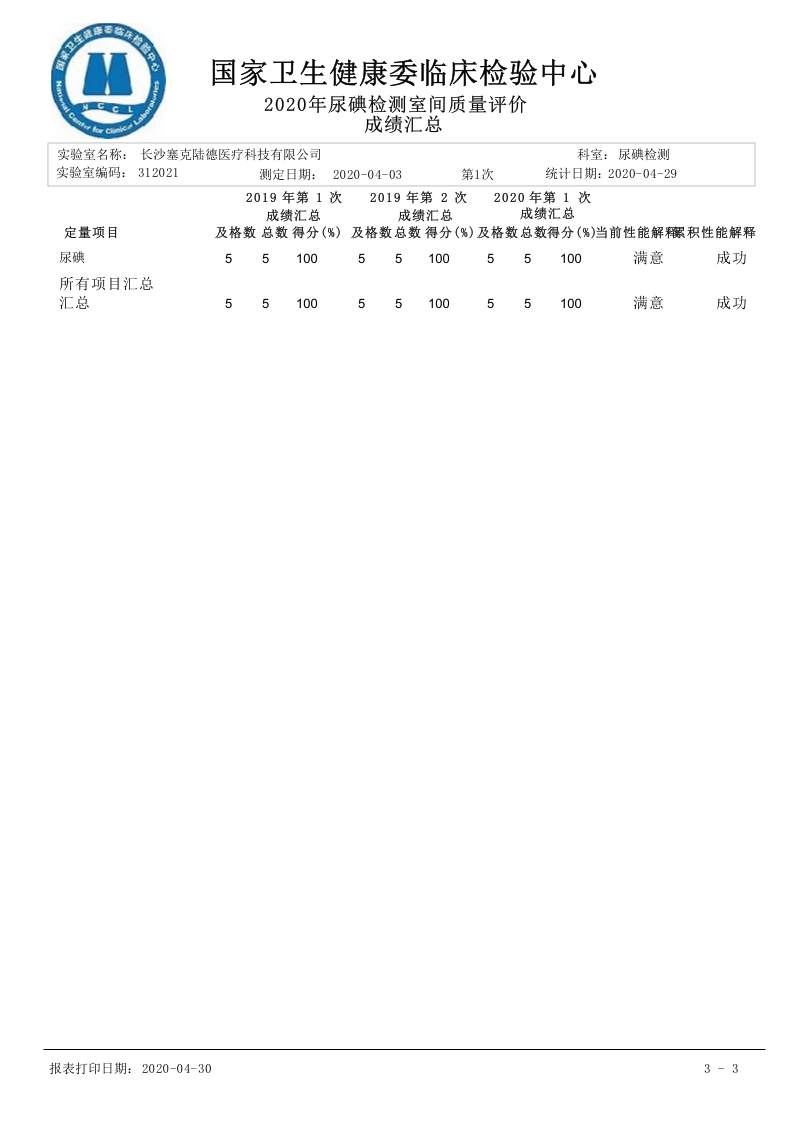
First Laboratory Quality Assessment Report
...
-
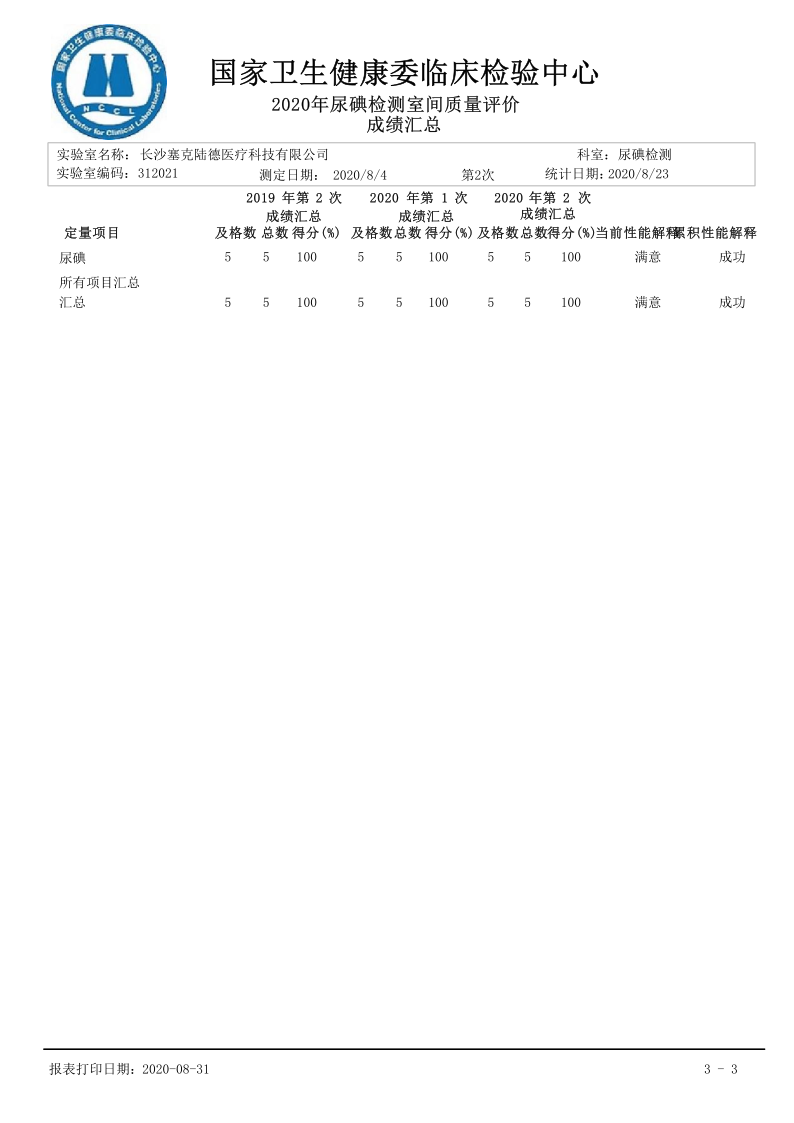
Second Laboratory Quality Assessment Report
...
-

Production License
...
-

Equipment Registration Certificate
...
-

Second Generation Equipment Registration Certificate
...
-
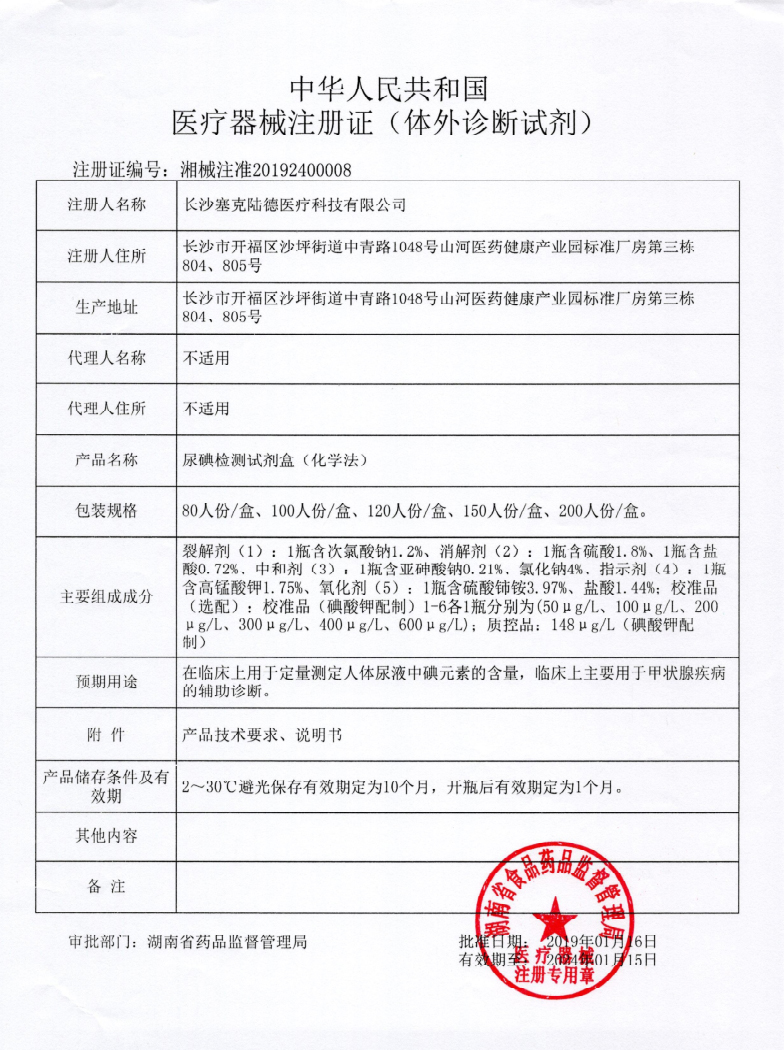
Reagent registration certificate
...